Art in Chemistry
For years we’ve heard that students have multiple intelligences and that we should do whatever possible to help them display their learning strengths using varied instructional strategies. While chemistry can be an abstract and quantitative science, there is much room for students to display their creativity while engaging multiple levels of conceptual understanding.
One of my favorite activities is a simple demonstration in which a small amount of water is heated in a flask until it is boiling, the heat is then turned off, and an inflated balloon is used to cover the opening. As the steam condenses inside the flask, the balloon moves inside the flask, and a vacuum is formed. Eventually, the vacuum is strong enough to lift the entire flask with just the balloon. The best part of this demonstration is what happens next. Students are asked to create a four-panel cartoon in which the molecules of air, water and steam must move and talk to explain what they are doing on the particle level and how that affects what we see on the macroscopic level (the real world). Students then participate in a silent gallery walk offering written commentary on each other’s art. This is a common example of modeling in chemistry nowadays (using particulate models) with a twist. The cartoon elements allow for some pretty interesting scenarios. One of my students decided to describe the movement of particles using a TV sportscaster analogy.
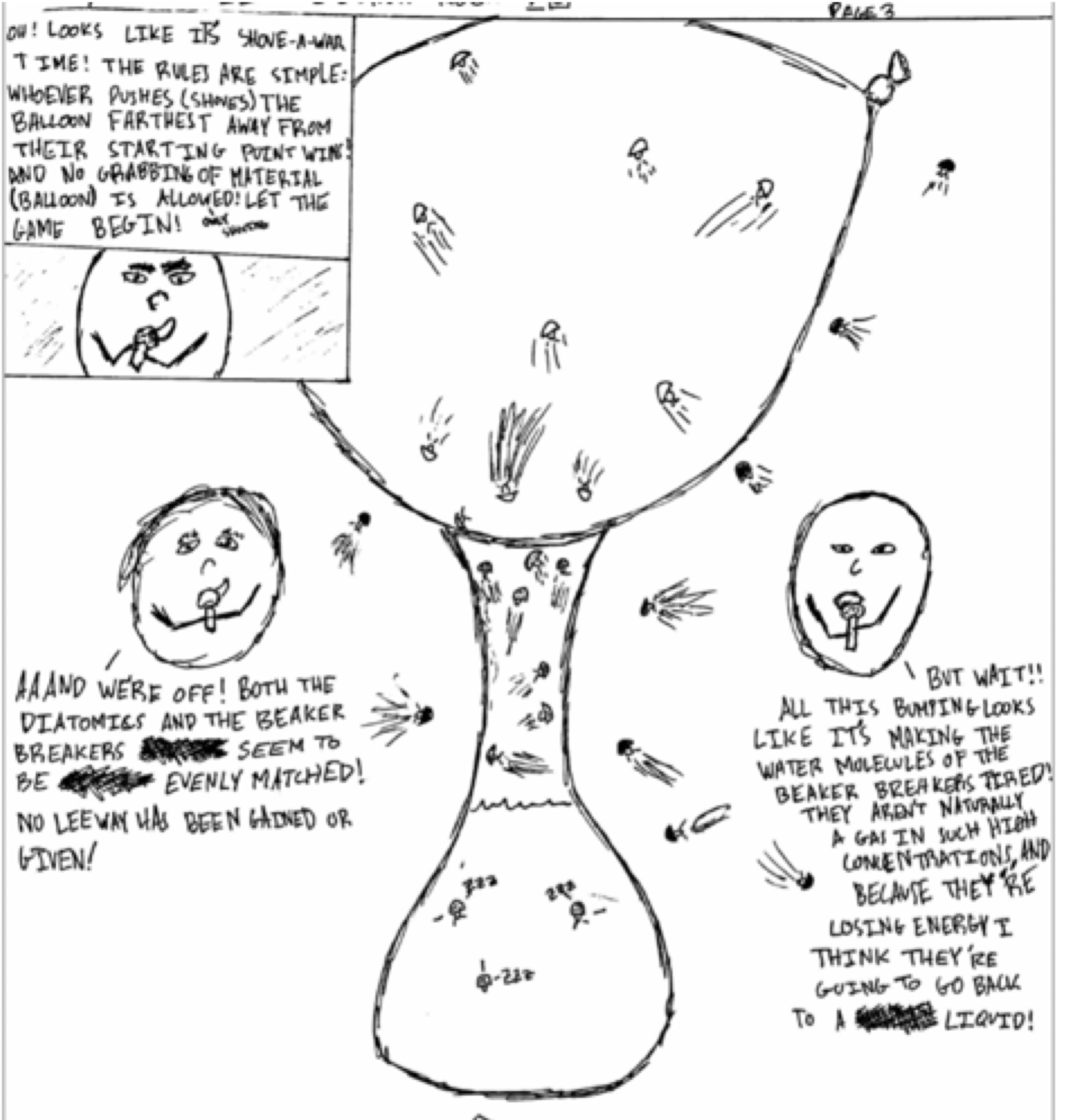
His cartoon correctly explained why the balloon moved inside the flask (he didn’t fall into the “flask molecules are sucking the balloon molecules” trap), and he did so with a deep conceptual understanding in a fun, creative manner.
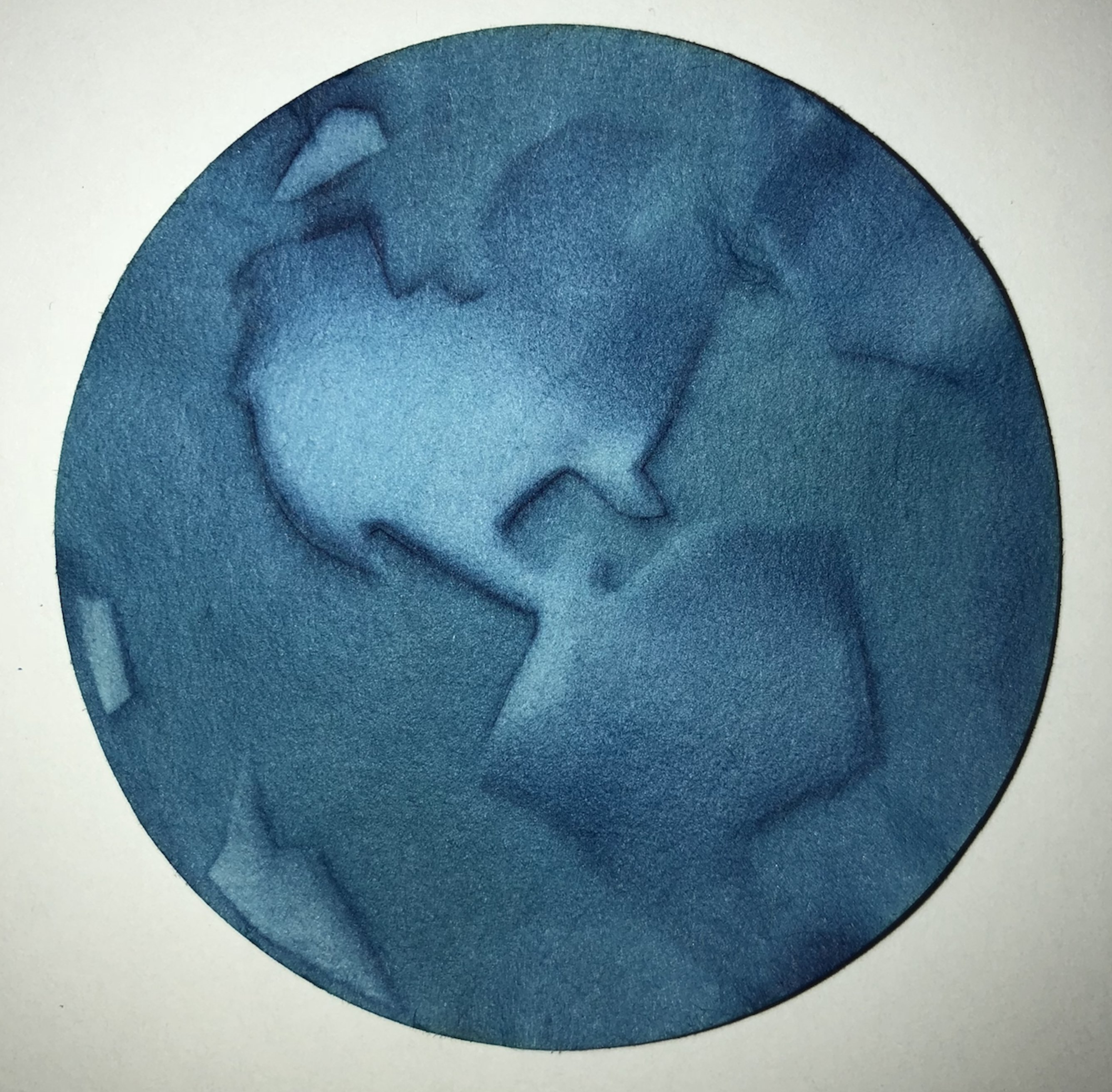
After the AP® Chemistry exam, I try to engage my students in many chemistry-based projects focusing on their artistic side. For example, after studying electrochemistry, we soak some goldenrod paper in salt water, place it on aluminum foil and use a copper wand connected to a 9-volt battery to create an image using the goldenrod paper as an acid-base indicator (check out the Goldenrod Electrochemistry Kit at teachersource.com). The students achieved some stunning results. My favorite one is of the eye pictured below. This student learned how to use this medium in just one try.
 |  |  |
Another lab involved the science of blueprinting. Students soak filter paper in a few solutions and prepare some masking using a paper plate. They cover the paper with their masking and develop the blueprints outside using ultraviolet light from the sun. You’ll find this lab at flinnsci.com (AP6155). Again, some amazing results are obtained using a fairly complex chemical system.
 |  |  |
Finally, I have students make Shrinky Dinks® using a non-traditional method. This was a popular toy back in my childhood. It involves drawing an image with permanent marker on a piece of polystyrene plastic and then putting it in the oven on low heat. You watch the plastic curl up in front of your eyes and end up with a smaller, thicker version of what went in. Instead of using an oven, students were armed with heat guns and crucible tongs and had to heat their plastic design slowly and carefully. The curling that takes place during heating is unpredictable and quite insane, but (almost) always ends up in a unique, smaller version of their artwork. (Heat guns and polystyrene sheets can be purchased online by the teacher.) Patience and skill must be practiced and cultivated by the students. Here are a few of my favorite designs.
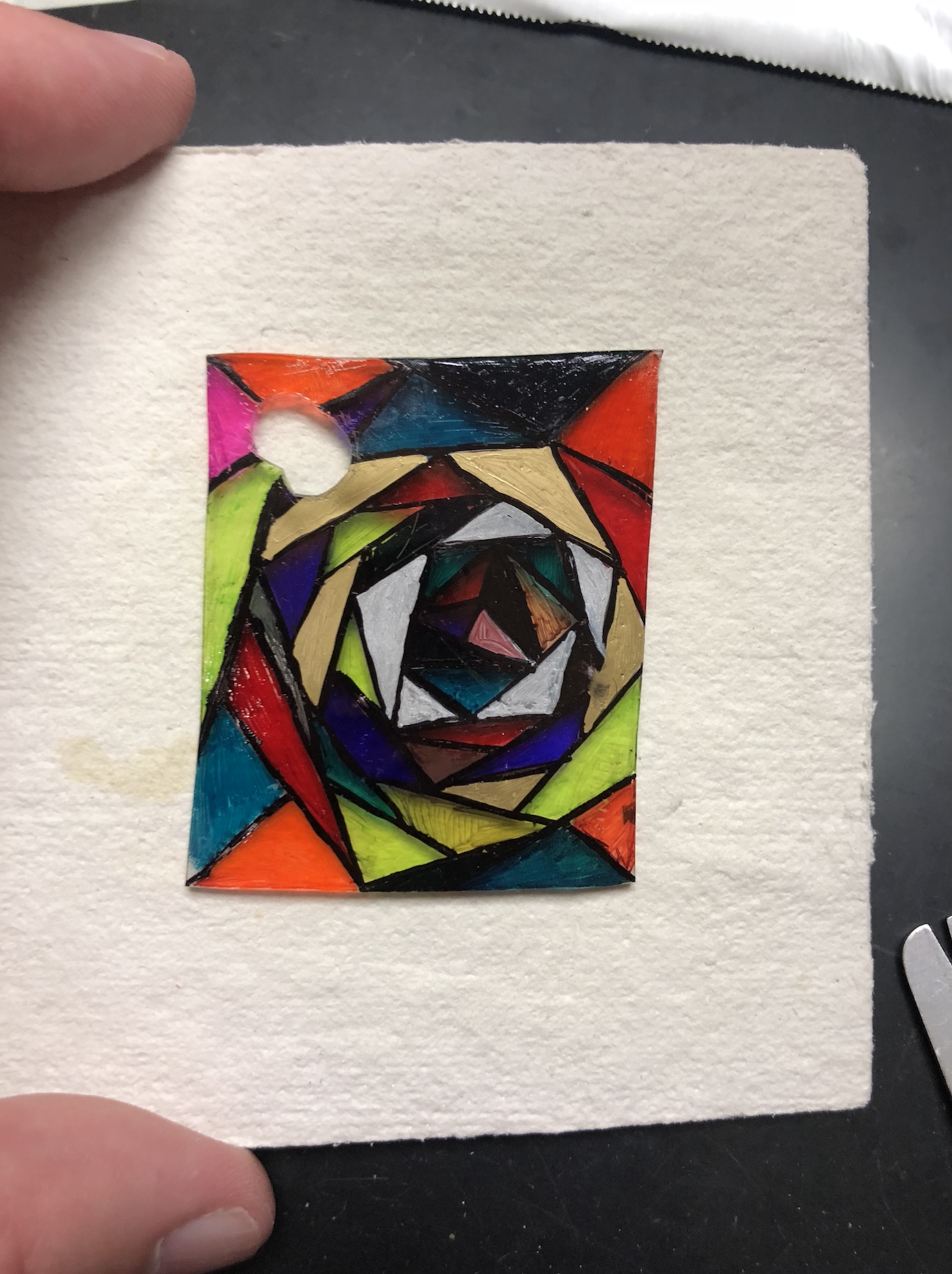 |  |  |
Hopefully you have been artistically inspired to try out some creative projects with your students this school year. Have fun!
*AP® is a trademark
registered by the College Board, which is not affiliated with, and does not
endorse, TI products. Policies subject to change. Visit www.collegeboard.com.
Shrinky Dinks is a
registered trademark of its respective owner and is not affiliated with, nor
endorsed by Texas Instruments.
Tagcloud
Archive
- 2024
-
2023
- January (3)
- February (3)
- March (5)
- April (3)
- May (3)
- June (3)
- July (2)
-
August (6)
- 5 Ways to Spruce Up Your Classroom for Back to School
- Day of the Dog: Which Dog Is Roundest?
- Women Who Code: A TI Intern’s Fascinating STEM Journey
- 6 Sensational TI Resources to Jump-Start Your School Year
- 3 Back-to-School Math Activities to Reenergize Your Students
- A New School Year — A New You(Tube)!
- September (2)
- October (3)
- November (2)
- 2022
-
2021
- January (2)
- February (3)
- March (5)
-
April (7)
- Top Tips for Tackling the SAT® with the TI-84 Plus CE
- Monday Night Calculus With Steve Kokoska and Tom Dick
- Which TI Calculator for the SAT® and Why?
- Top Tips From a Math Teacher for Taking the Online AP® Exam
- How To Use the TI-84 Plus Family of Graphing Calculators To Succeed on the ACT®
- Celebrate National Robotics Week With Supervised Teardowns
- AP® Statistics: 6 Math Functions You Must Know for the TI-84 Plus
- May (1)
- June (3)
- July (2)
- August (5)
- September (2)
-
October (4)
- Transformation Graphing — the Families of Functions Modular Video Series to the Rescue!
- Top 3 Halloween-Themed Classroom Activities
- In Honor of National Chemistry Week, 5 “Organic” Ways to Incorporate TI Technology Into Chemistry Class
- 5 Spook-tacular Ways to Bring the Halloween “Spirits” Into Your Classroom
- November (4)
- December (1)
-
2020
- January (2)
- February (1)
- March (3)
- April (1)
- May (2)
- July (1)
- August (2)
- September (3)
-
October (7)
- Tips for Teachers in the time of COVID-19
- Top 10 Features of TI-84 Plus for Taking the ACT®
- TI Codes Contest Winners Revealed
- Best of Chemistry Activities for the Fall Semester
- Best of Biology Activities for the Fall Semester
- Best of Middle Grades Science Activities
- Best of Physics Activities for the Fall Semester
- November (1)
- December (2)
- 2019
-
2018
- January (1)
- February (5)
- March (4)
- April (5)
- May (4)
- June (4)
- July (4)
- August (4)
- September (5)
-
October (9)
- Art in Chemistry
- Which Texas Instruments (TI) Calculator for the ACT® and Why?
- Meet TI Teacher of the Month: Jessica Kohout
- Innovation in Biology
- Learning With Your Students
- A first-of-its-kind STEM strategy charts path to help educators
- #NCTMregionals Hartford 2018 Recap
- The Math Behind “Going Viral”
- Real-World Applications of Chemistry
-
November (8)
- Testing Tips: Using Calculators on Class Assessments
- Girls in STEM: A Personal Perspective
- 5 Teachers You Should Be Following on Instagram Right Now
- Meet TI Teacher of the Month: Katie England
- End-of-Marking Period Feedback Is a Two-Way Street
- #NCTMregionals Kansas City 2018 Recap
- Slope: It Shouldn’t Just Be a Formula
- Hit a high note exploring the math behind music
- December (5)
- 2017
- 2016
- 2015
